
/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
The lead(II) chloride becomes even less soluble, and the concentration of lead(II) ions in the solution decreases. Of course, the concentration of lead(II) ions in the solution is so small that only a tiny proportion of the extra chloride ions can be converted into solid lead(II) chloride. Strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure. What happens to that equilibrium if extra chloride ions are added? According to Le Châ telier, the position of equilibrium will shift to counter the change, in this case, by removing the chloride ions by making extra solid lead(II) chloride. The sum of the oxidation states within a compound or ion must equal the overall charge. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. The common or trivial names are somewhat old fashioned nowadays, but they're still used in some places, so they're helpful to know. For some ions, the common or trivial name is also given.
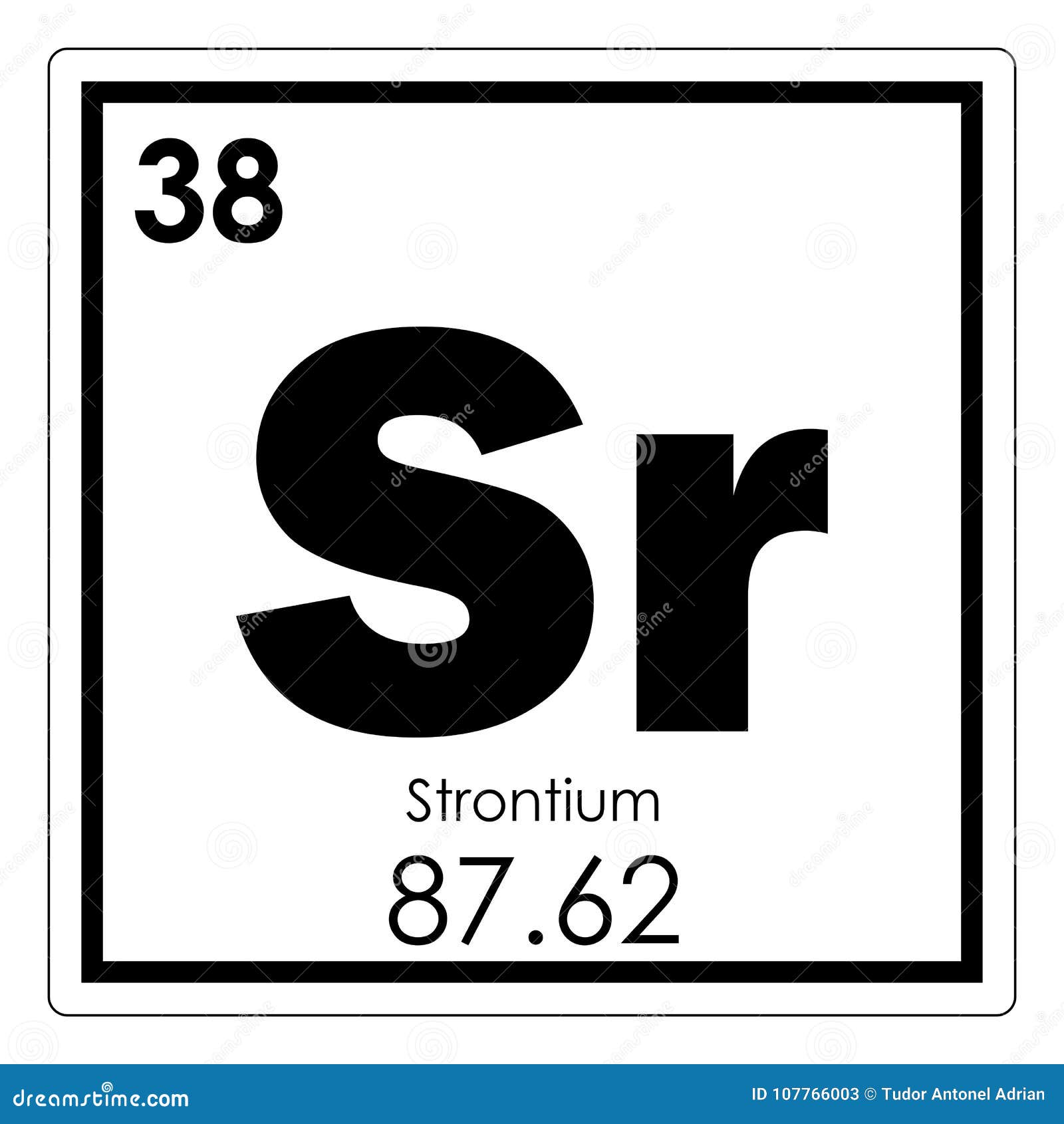
The systematic name is included for all ions. Similarly, as we proceed across the row, the increasing nuclear charge is not effectively neutralized by the electrons being added to the 2 s and 2 p orbitals.\ (aq) + 2Cl^- \ (aq)\nonumber \] The oxidation state of an atom is a measure of the degree of oxidation of an atom. The following table lists some of the most common ions for polyvalent metals. The metal forms a dark oxide layer when it is exposed to air. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. Question: What is the charge of the common ion formed by each of these atoms Either -2,-1,+1, or +2. Strontium is the chemical element with the symbol Sr and atomic number 38. Consequently, beryllium is significantly smaller than lithium. Youll get a detailed solution from a subject matter expert that helps you learn core concepts. This means that the effective nuclear charge experienced by the 2 s electrons in beryllium is between +1 and +2 (the calculated value is +1.66). (More detailed calculations give a value of Z eff = +1.26 for Li.) In contrast, the two 2 s electrons in beryllium do not shield each other very well, although the filled 1 s 2 shell effectively neutralizes two of the four positive charges in the nucleus. Thus the single 2 s electron in lithium experiences an effective nuclear charge of approximately +1 because the electrons in the filled 1 s 2 shell effectively neutralize two of the three positive charges in the nucleus. Although electrons are being added to the 2 s and 2 p orbitals, electrons in the same principal shell are not very effective at shielding one another from the nuclear charge. All have a filled 1 s 2 inner shell, but as we go from left to right across the row, the nuclear charge increases from +3 to +10. Which of these metals will form ions most readily and why. The atoms in the second row of the periodic table (Li through Ne) illustrate the effect of electron shielding.
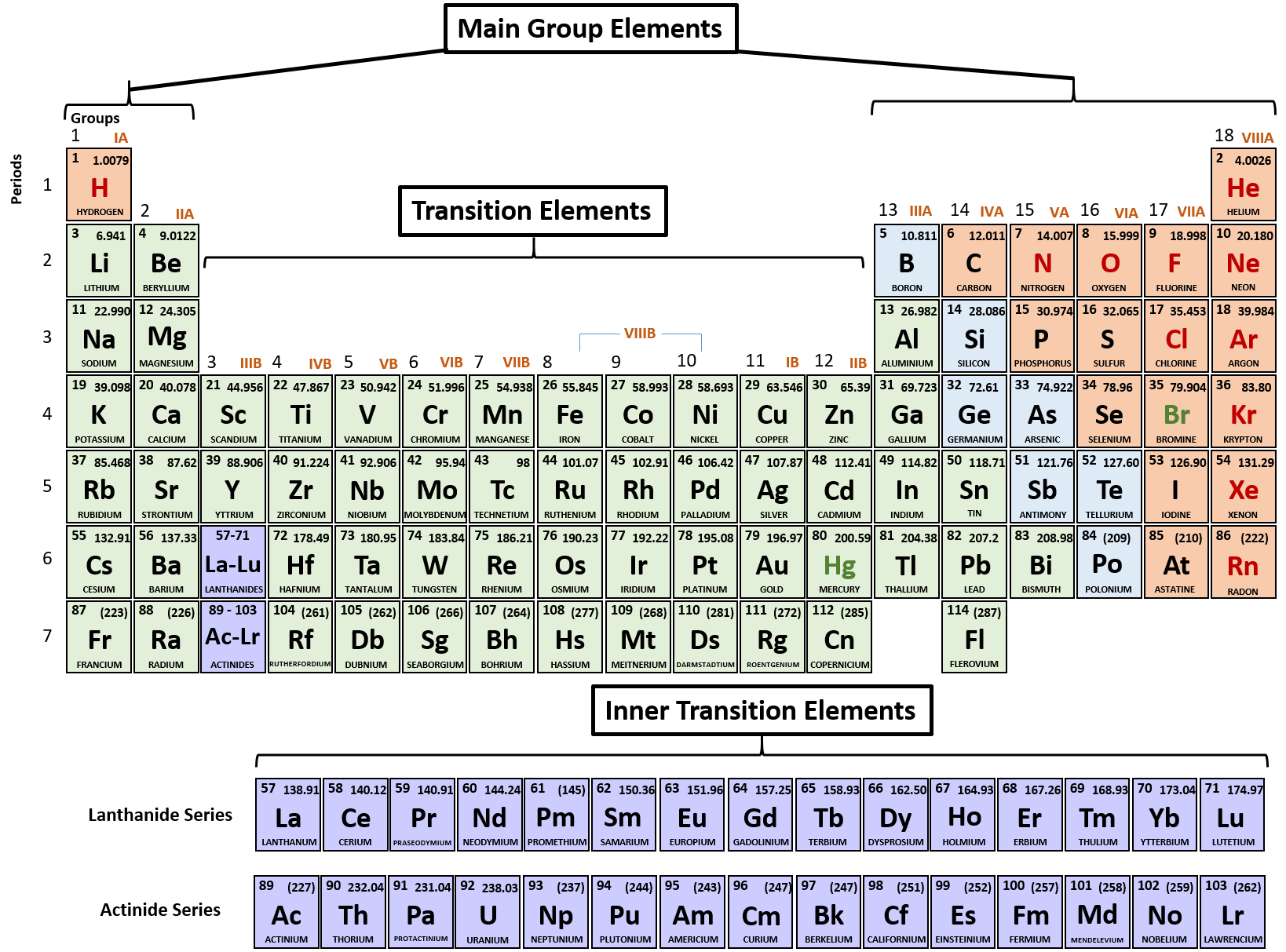
The atomic number of each element increases by one, reading from left to right. most common oxidation states of iron are Fe2+, the ferrous or iron(II) ion. Period A horizontal row in the periodic table. Information about various chemical compounds and elements. We will assume that these elements form ions that have the same number of electrons as the nearest. Members of a group typically have similar properties and electron configurations in their outer shell. The strontium-90 isotope has 90 38 52 neutrons.

The greater the effective nuclear charge, the more strongly the outermost electrons are attracted to the nucleus and the smaller the atomic radius.Ītomic radii decrease from left to right across a row and increase from top to bottom down a column. Strontium Sr Strontium 38 87.62 Glossary Group A vertical column in the periodic table. For all elements except H, the effective nuclear charge is always less than the actual nuclear charge because of shielding effects. \( \newcommand\)) experienced by electrons in the outermost orbitals of the elements.


 0 kommentar(er)
0 kommentar(er)
